Flagship-founded companies joined other leaders in cell and gene therapy at last week’s ASGCT Annual Meeting in Baltimore, MD, sharing strides in the development of their respective bioplatform technologies and progress toward potentially transformative medicines. The conference felt abuzz as the sector demonstrated breakthrough potential in a range of indications and regulators shared efforts to enhance the regulatory process. For the Flagship ecosystem, this was an exciting moment to share pioneering work in genetic medicines and cell therapy from Ring Therapeutics, Tessera Therapeutics, Alltrna, Omega Therapeutics, and Sana Biotechnology.
Ring Therapeutics
Ring shared data in nonhuman primates showing the ability to redose genetic medicines using their anellovector platform. In an interview with BiotechTV, Ring CEO and Flagship CEO-Partner Tuyen Ong said that the ability to redose genetic medicines would prevent patients and caregivers from having to accept tradeoffs when pursuing treatment as the efficacy of genetic cargos evolve. Watch the full interview here on BiotechTV here and listen to a conversation with Tuyen and Flagship Origination Partner and Ring co-founder Avak Kahvejian on about the company's journey turning an obscure virus into a redosable vector for genetic medicine.

Tessera Therapeutics
Tessera also shared preclinical data using its Gene Writers and proprietary lipid nanoparticles to restore normal hemoglobin production in hematopoietic stem cells in the bone marrow, critically without chemotherapy or mobilization, for the treatment of sickle cell disease. In an interview on BiotechTV, Tessera CEO and Flagship CEO-Partner Mike Severino said this therapy could reduce the burden on patients with a simple IV infusion and increase access to these therapies to the 6 million sickle cell patients around the world. Watch the full interview here, and learn more about additional data Tessera presented at the conference.
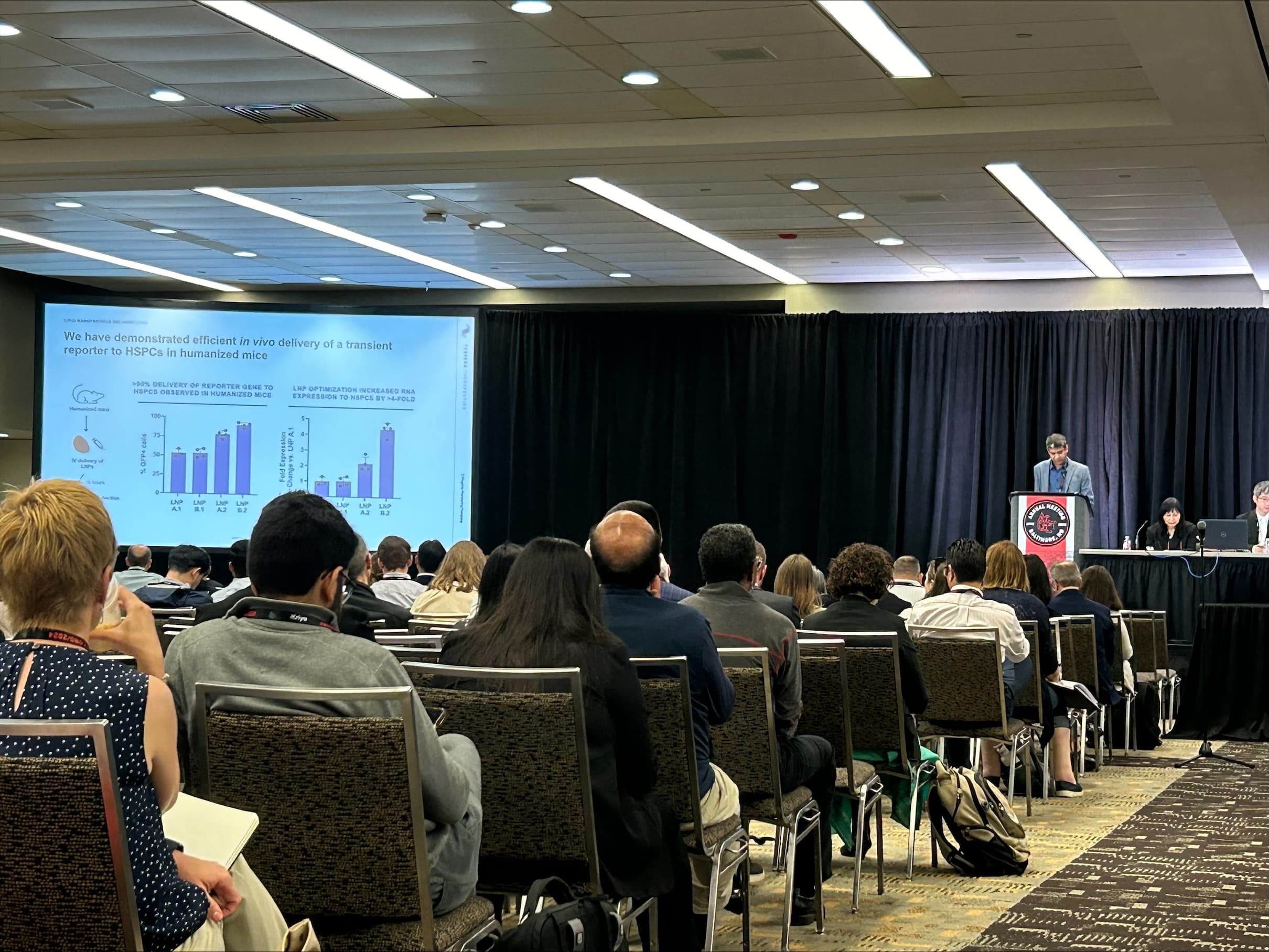
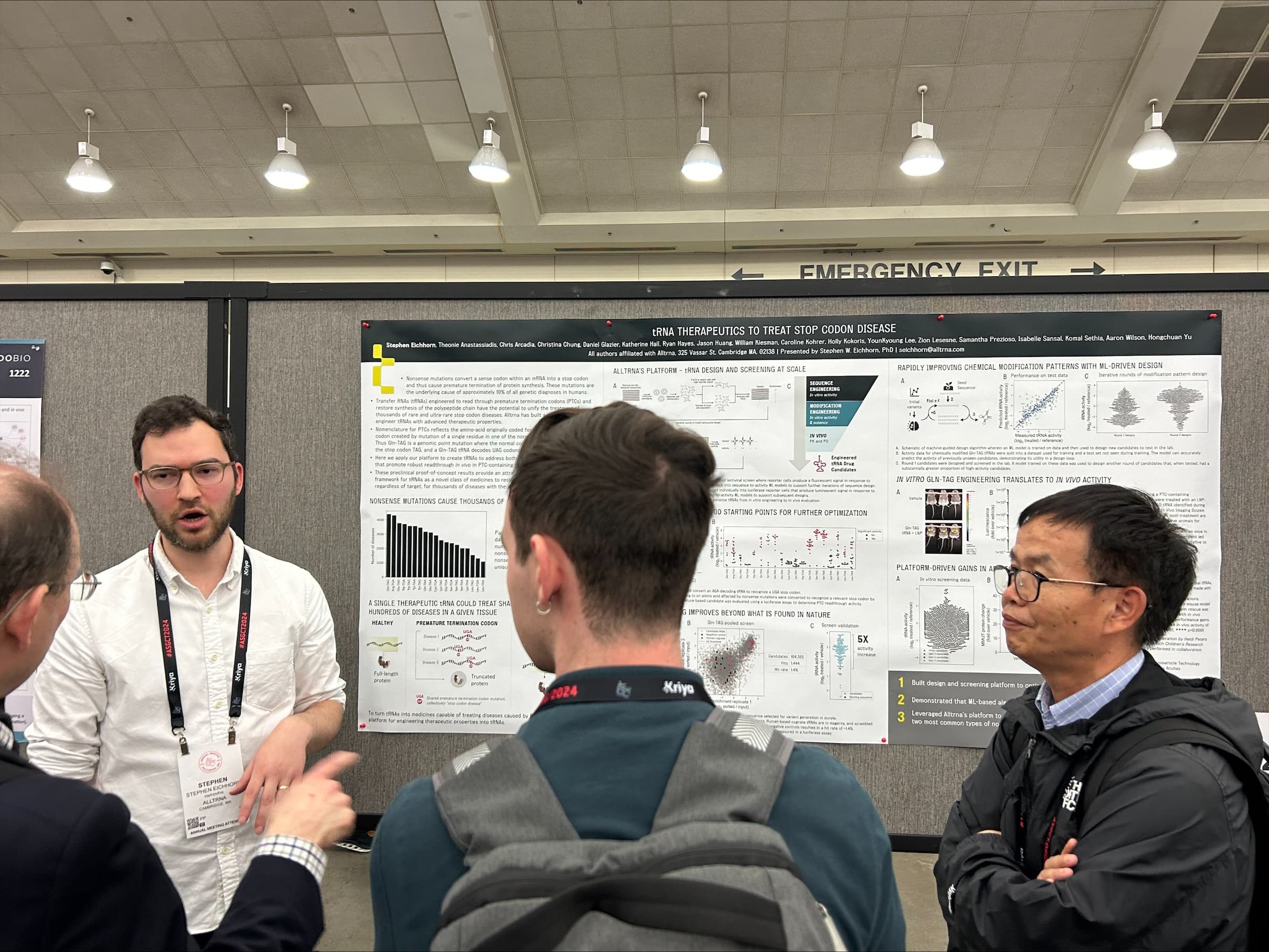
Alltrna
Alltrna, which is unlocking tRNA biology to treat disease, demonstrated robust ability to read through the two most common premature stop codons in genetic disease and rescue protein production in vivo. These promising results suggest Alltrna’s engineered tRNAs could provide new treatments for many genetic diseases, including the rare and ultra-rare diseases caused by nonsense mutations, known as stop codon disease.
Omega Therapeutics
Omega, a company pioneering the development of a new class of programmable epigenomic mRNA medicines, showed preclinical data highlighting its platform's ability to program epigenetic controllers to upregulate gene expression for a variety of genes types and cell conditions, providing effects that last weeks to months after a single dose.

Sana Biotechnology
Working to engineer cells as medicines, Sana presented data suggesting a promising safety profile for its in vivo CAR T therapy using the company’s fusogen technology for delivery of genetic payloads to CD8+ T cells. Christina Chaivorapol, VP of Translational Technologies, had an oral presentation outlining integration sites and genomic integrity comparing these T cell targeted therapies with the lentiviral delivery used for typical ex vivo manufactured CAR T therapies. Sana’s fusogen technology demonstrated the ability to generate in vivo CAR T cells with high specificity, targeted efficacy, and comparable genomic safety to ex vivo manufactured CAR T therapies. If successfully developed, this technology has the potential to meaningfully expand the number of patients that can benefit from CAR T cell therapies.

