Under a microscope, our cells look a lot like bustling production lines that are pumping out tiny molecular machines called proteins. These molecules are intricately designed and finely tuned to perform a vast array of functions to support life, from metabolizing food and fighting infections to enabling our interaction with the world through sight, hearing, and touch. However, just like any other factory, sometimes the gears grind to a halt. Premature termination codons, also called premature stop codons, are like tiny but disruptive glitches in our DNA that impede the smooth operation of our cellular machinery, leaving unfinished products and disrupted processes in their wake.
Across all genetic diseases, about ten percent of patients have premature termination codon mutations. But while these mutations may be present in thousands of diseases, current therapeutic development approaches to treat these disorders do not capitalize on this unifying factor. Instead, most medicines are developed by focusing on each individual disease or gene, a slow and complex process for addressing the more than 6,000 rare genetic diseases — leaving many patients without FDA-approved medicines or any treatment in development.
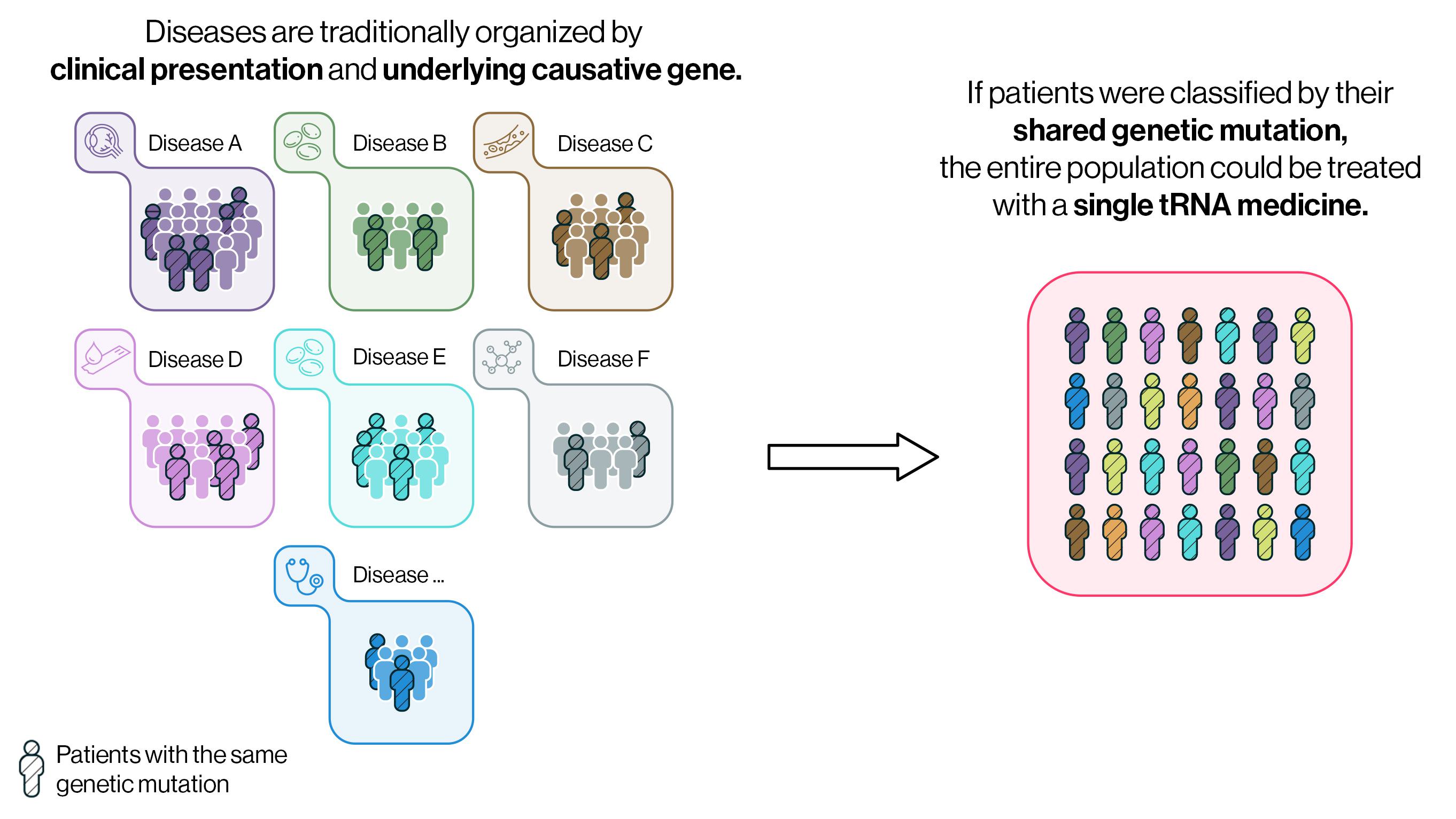
What if we could rethink this approach of classifying patients by their individual mutated gene or a traditionally defined clinical presentation and instead classify patients based on their shared genetic mutations? If so, patients with the same genetic mutation but seemingly disparate diseases, such as hemophilia, Duchenne muscular dystrophy (DMD), and cystic fibrosis (CF), could be classified under “stop codon disease” and treated with the same drug molecule.
The central dogma of biology
Understanding how premature termination codon mutations arise and the disruption they cause, requires a look at the intricate process of protein production. To begin, DNA is transcribed into messenger RNA (mRNA), which is made of a sequence of molecules called nucleotides, often called “bases.” Transfer RNA (tRNA) reads the mRNA three bases at a time (A, U, G, or C) as a “codon.” Each unique codon corresponds to one of 20 different amino acids (e.g., the codon ACA codes for the amino acid threonine). Different tRNAs physically pair with their complementary codon, shuttling the correct amino acid onto a growing polypeptide chain, which, when finished, will become the final protein.
However, certain codons are not read as amino acids but, instead, function as stop or termination codons. Three stop codons (UAG, UAA, and UGA) tell the cell’s machinery to halt mRNA translation and release the polypeptide chain. This translation termination process naturally occurs once the entire polypeptide is translated, enabling the formation of a stable and functional protein. But, if there is a mutation in the DNA that creates a premature stop or termination codon, the translation of the polypeptide is terminated before the protein is complete. The incomplete protein is typically not able to carry out its function, resulting in disease.
A paradigm shifting approach
Despite its critical role, the contributions of tRNA are often glossed over when detailing the protein production process. The Alltrna team recognized the importance of tRNA in this process and saw a way to revolutionize therapeutic development. Alltrna is engineering tRNAs to restore the assembly of proteins, identifying misplaced stop codons and patching in an amino acid fix. These novel therapeutics would recognize and bind to the premature termination codon, overriding the signal to stop translation and adding the correct amino acid at the corresponding site in the protein. These engineered tRNAs would allow generation of the full-length standard protein, treating disease at the site of protein production instead of altering the cell’s DNA or mRNA.
Because of the finite combinations of amino acids and stop codons, it would require fewer than 20 engineered tRNAs to treat all the patients with stop codon disease — any genetically driven disease that stems from a premature termination codon.
Because of the finite combinations of amino acids and stop codons, it would require fewer than 20 engineered tRNAs to treat all the patients with stop codon disease — any genetically driven disease that stems from a premature termination codon. This would revolutionize how therapeutics are developed for the 6,000+ genetically defined rare diseases. Today, only 5% of rare genetic diseases have FDA-approved medicines and, due to small patient populations, many are left without any drugs in development. Because Alltrna’s engineered tRNAs could be used for any patient with a premature termination codon, access to life-saving medicines can be extended to patients with a broad range of diseases much more quickly than the slow process of developing medicines for each individual disease.
Accelerating treatments for all patients with stop codon disease
There is no question that we need a new approach to developing drugs for genetically driven diseases, as the current paradigm is slow and exclusionary. Opportunities where we can identify patients with similar mutations and treat them with the same drug regardless of their disease will accelerate our ability to address the large number of genetic diseases. Alltrna is excited by the potential of tRNA medicines and the life-changing impact they could have on the approximately 30 million people with stop codon disease.

