- Genetics have transformed drug discovery and development, enabling the biologics revolution that has impacted millions of patients and created billions of economic value.
- The same paradigm behind biologics can be used to discover and develop small molecule medicines from microbial DNA found within the human body, effectively creating therapeutics that combine the benefits of both modalities.
- Empress Therapeutics is leveraging genetic chemistry to source chemistry from within the human body. This approach increases speed and certainty in small molecule drug discovery and reveals new ways to treat diseases.
Biologics have proved that inside the human body exists a rich source of bioactive molecules. These therapeutics are derived from living organisms or their components and include examples such as the protein insulin for diabetes management, monoclonal antibodies used as cancer therapies, mRNA vaccines against SARS-CoV-2, and CRISPR/Cas9-based gene editing therapies for sickle cell disease. These and other biologic medicines have led to treatments for millions of patients and created billions in economic value.
Because the instructions for building these molecules are inscribed in DNA, the design and production of many biologics can be rationally programmed along the central dogma of biology: DNA to mRNA to protein. Not only does this speed production, but it also increases the likelihood that the resulting molecules are compatible with our biology and, therefore, more likely to be safe and effective.
Our ability to plug into this linear progression is the foundation of the biotech industry we know today. The production of insulin using recombinant DNA is widely regarded as the dawn of biotech. This hormone responsible for keeping blood sugar in check was found to positively impact diabetes symptoms in the 1920s. But up until the 1970s, the process for producing therapeutic insulin involved harvesting and processing huge quantities of animal pancreases. The discovery of the structure of DNA in the interim informed the development of recombinant DNA methods, in which a DNA sequence is inserted into the genome of bacteria to be expressed as a synthetic version of a human protein like insulin. This turning point showcased the rational design and production of safe and effective treatments at scale using genetic engineering.
And while biotech began with biology, we have found that the power and promise of genetics can now be extended to chemistry.
Small molecules with big benefits
Overlooked in this biological revolution were small molecules. Historically a pillar of traditional medicine, these synthetic medicinal chemicals are packaged into pills, and include familiar molecules such as statins for cholesterol management and antibiotics like penicillin, as well as more recently approved drugs such as Evrysdi for spinal muscular atrophy and Trikafta for cystic fibrosis.
Clinical success rates for small molecules have lagged behind biologics. Unfortunate, because small molecules offer significant advantages to patients and healthcare systems. Chemical compounds can reach disease targets inside of cells and throughout the entire body in a way that most biologics cannot. Moreover, they offer flexibility in administration; orally available drugs are convenient for patients to take and prescribers to administer, and they can be easily made and distributed at scale using established pharmaceutical manufacturing methods, which makes them typically much more affordable than other drug modalities.
In short, chemistry makes great drugs. Yet, conventional small molecule drug discovery has long been an unpredictable, lengthy, difficult process. Designing and testing these molecules has required trial and error and laborious chemical synthesis to create precise chemical structures, often only to produce experimental medicines that do not successfully modulate disease or have serious safety concerns.
Borrowing from biologics
Biosynthesis, in which organisms build complex molecules from simpler ones, is a fundamental feature of every living cell, from bacteria, to plants, to mammals. In addition to the larger molecules we call biologics, cells produce small molecules, too. Enzymatic proteins encoded in clusters of genes work together to make and modify bioactive small molecules.
As a result, the central dogma can be extended beyond proteins to small molecules, enabling the use of genetic code to generate medicines based on chemistry. The result is a seemingly ideal drug — one that combines the versatility of small molecules with the inherent human relevance and origins in genetics-based programmability of biologics.
Mining microbial DNA for the foundations of medicines
Within the human body, the trove of microbial DNA is a logical source for the beginnings of small molecule medicines. This chemical universe produced by microbes inside each of us represents the sum result of more than 300,000 years of evolutionary experimentation — untold quadrillions of variations, tested across 100 billion human lives. It is as though these molecules have undergone colossal clinical trials on an unimaginable scale through the mechanism of evolution itself. As such, these microbial-made molecules are compatible with human biology and, because they have been conserved across millennia, likely have roles in health and disease.
This is where Empress Therapeutics is beginning its search for the foundations of new drugs. From human samples, we can pinpoint the biosynthetic gene clusters in DNA that give rise to these molecules. This process reveals the DNA instructions for small molecule drug leads, which we can then match to disease-relevant targets.
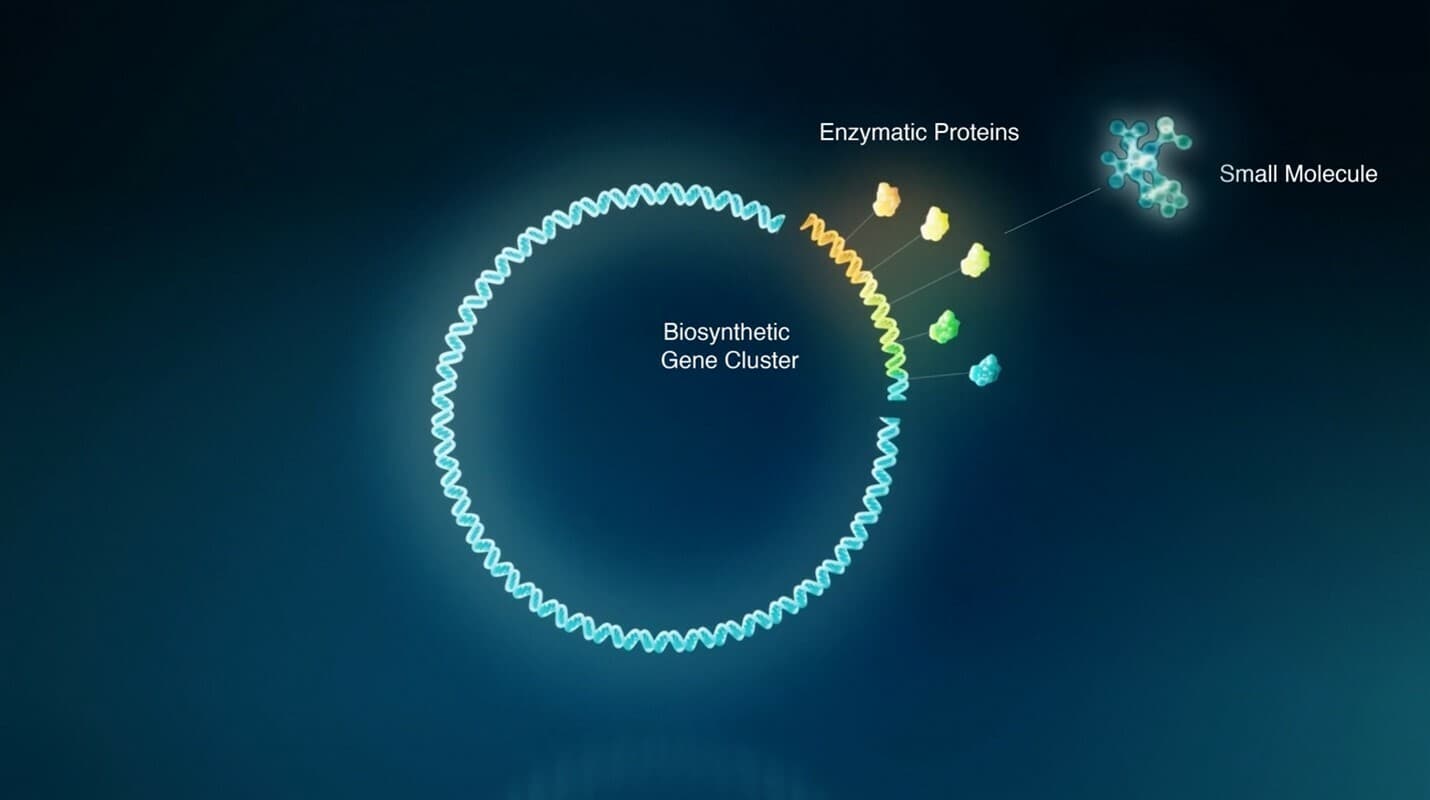
Instead of synthesizing a new small molecule from scratch, Empress can recode these genetic programs using synthetic biology and program engineered bacteria to produce chemical compounds, which serve as the foundation of modified small molecules with increased accessibility and targeted biological profiles. We call this approach genetic chemistry — the use of genetic code to discover the foundations of small molecule medicines.
Increasing the speed and certainty of small molecule drug discovery
Empress has already identified compounds that span multiple structural classes and target multiple classes of proteins. These compounds have the potential to become first-in-class or best-in-class medicines to address areas of high unmet needs, including immune and inflammatory, metabolic, neurologic, oncologic, and pain disorders.
For those impacted by disease, the need for medicines that are safe, effective, and accessible is fundamental. But for too many diseases, medicines that meet these needs are not available. Empress is working to increase the speed and certainty with which we can deliver promising new medicines and make an impact for patients, physicians, families, and caregivers.

