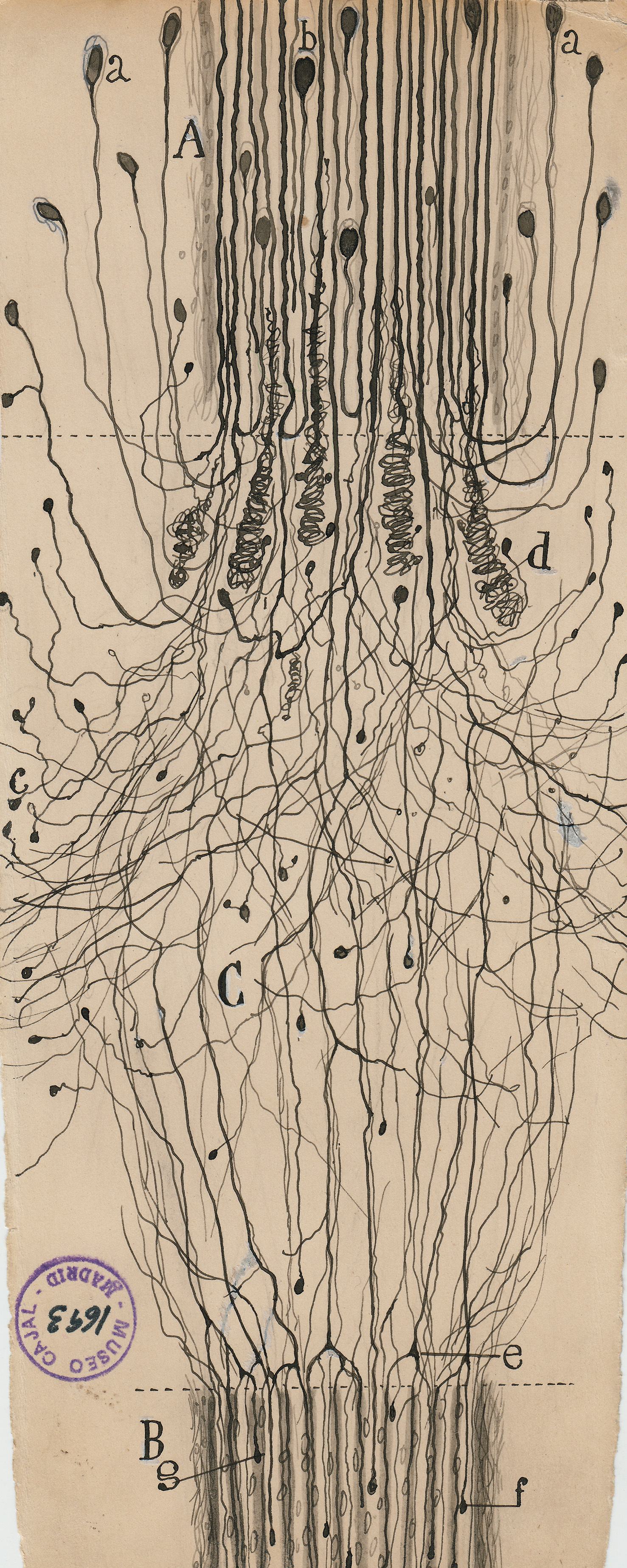
The curse of modern brain science is that its founding father, Santiago Ramón y Cajal, was an incurable artist.
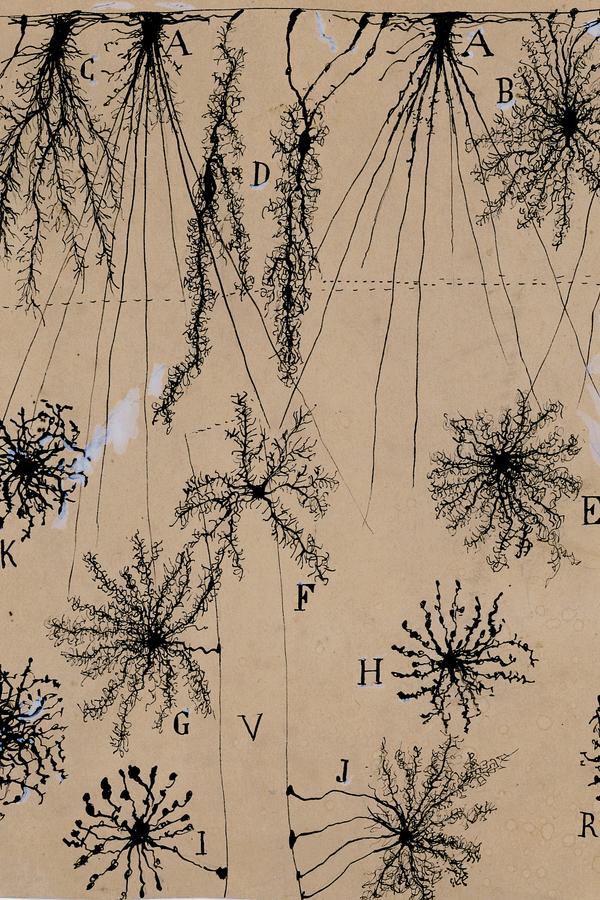
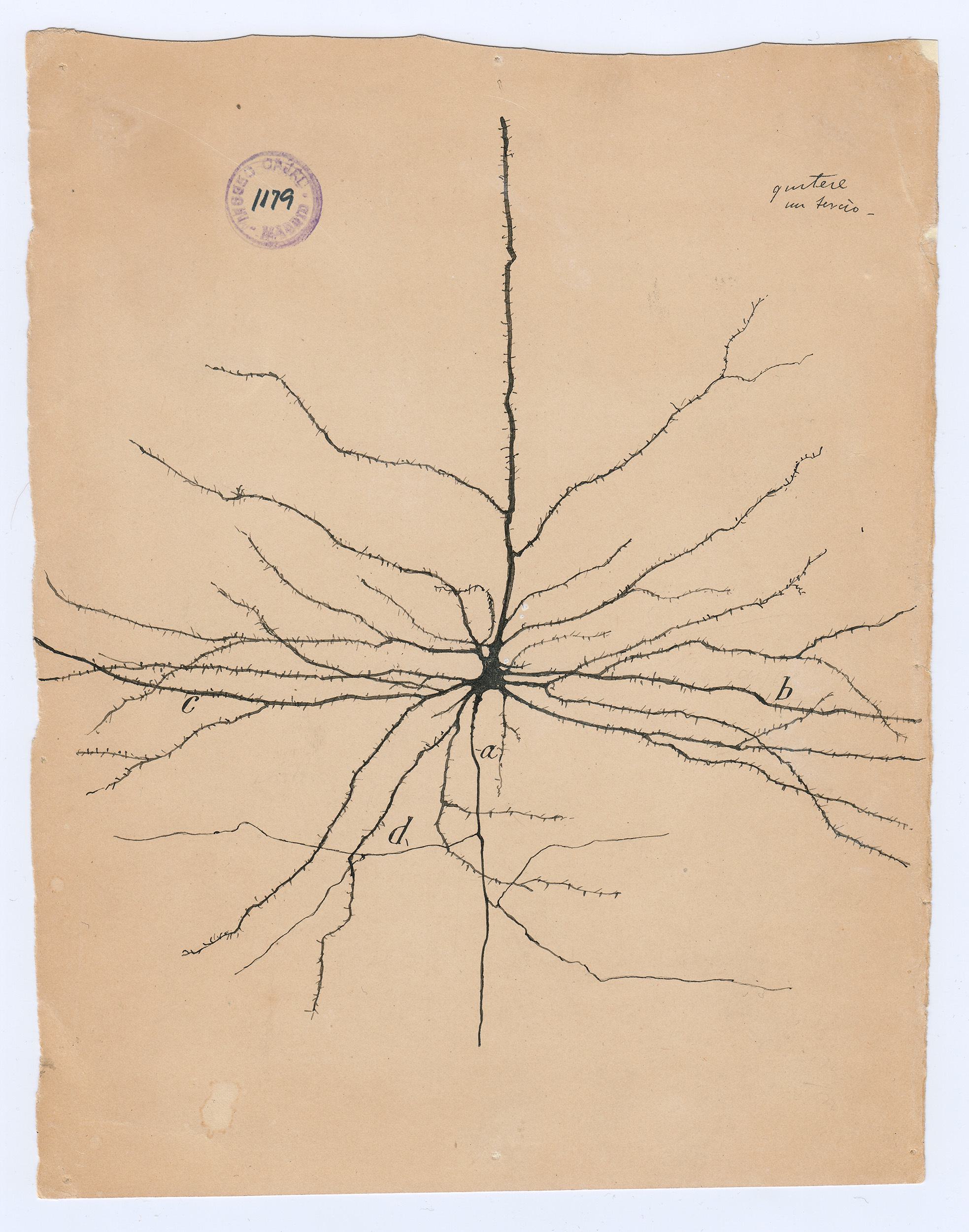
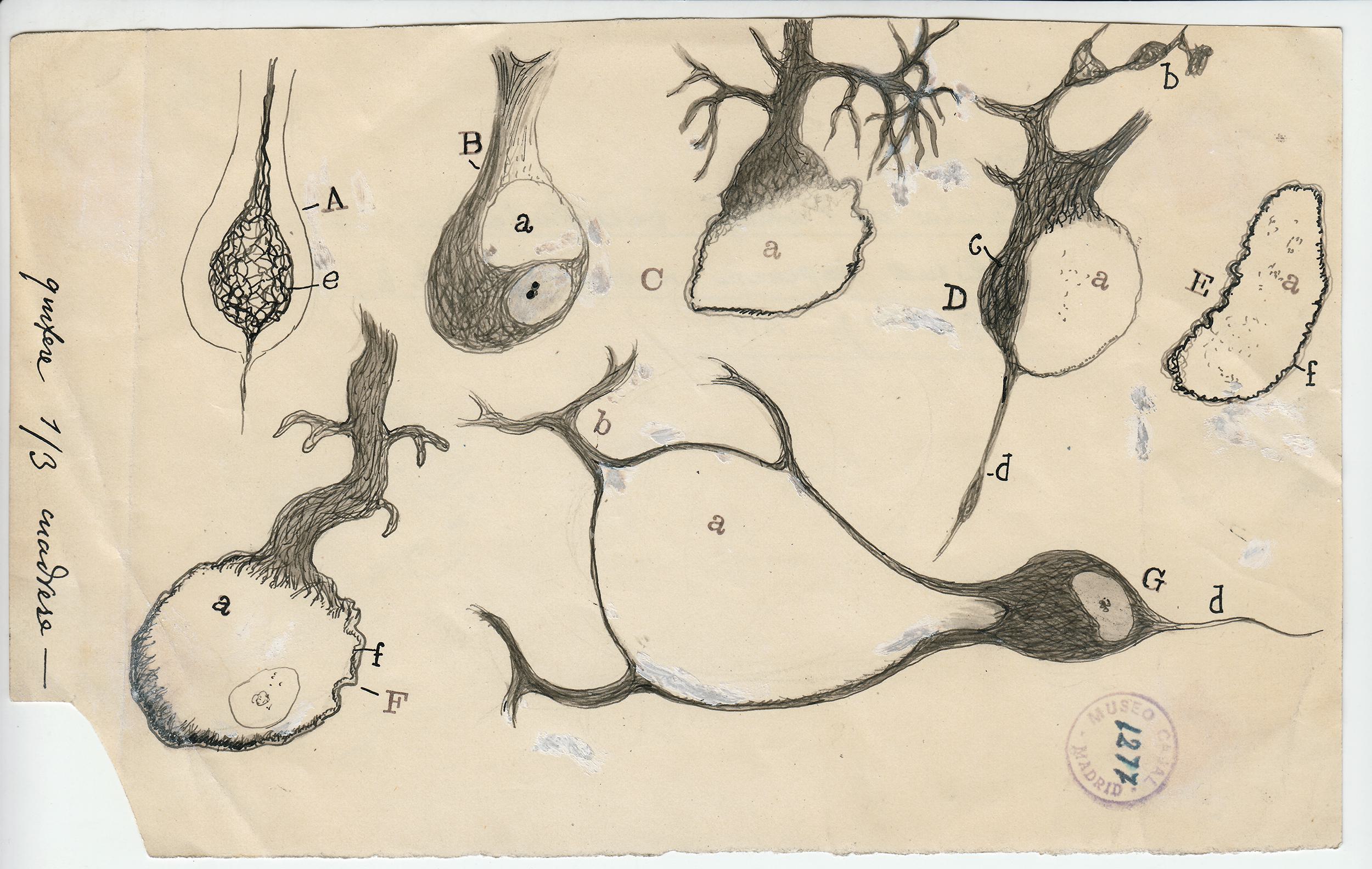
The Spanish neuroscientist felt that to see an object properly—say, a Purkinje neuron, with its branches as luxurious as a sea fan’s—he needed to draw it. But while his freehand pencil-and-ink sketches have mesmerized students of the brain for decades, they were not exact depictions of the cells he had so painstakingly stained and fixed under his microscope. They were interpretations; they were arguments. Cajal drew not just to see but to explain.
Today, more than 80 years after Cajal’s death, neuroscientists have traded their pencils for electron microscopes and MRI machines. These new tools also produce mesmerizing, multi-colored, often computer-animated images—pictures that have fueled countless scientific papers and popular accounts of progress in brain science. But they represent a looming trap.
Cajal’s task was to work out the basic microanatomy of the nervous system. He was the first to show that the brain contains billions of discrete cells, rather than an undifferentiated web of neural tissue.
Today’s neuroscientists are ready to probe deeper kinds of questions. Ultimately, they’d like to explain how perception, thought, and memory work. They want to know how “the flower garden of the gray matter” becomes a habitat for “the mysterious butterflies of the soul,” as Cajal framed the question in his autobiography. Ultimately, though, those are questions about function and integration, not just structure, and the explanations will not be found in images alone.
To visit The Beautiful Brain—a traveling exhibition of 80 of Cajal’s original drawings that landed in the fall of 2018 at the MIT Museum in Cambridge, Massachusetts—is to understand immediately why neuroscience, from its earliest moments, fell under the spell of visualization. Cajal’s drawings are diminutive, often no more than a few inches on a side (the museum supplies visitors with magnifying glasses). But they are alive with texture, depth, and whimsy. The viewer relates to Cajal’s delicate renderings of pyramidal neurons, astrocytes, and blood cells as if they were characters in a graphic novel about cellular survival.
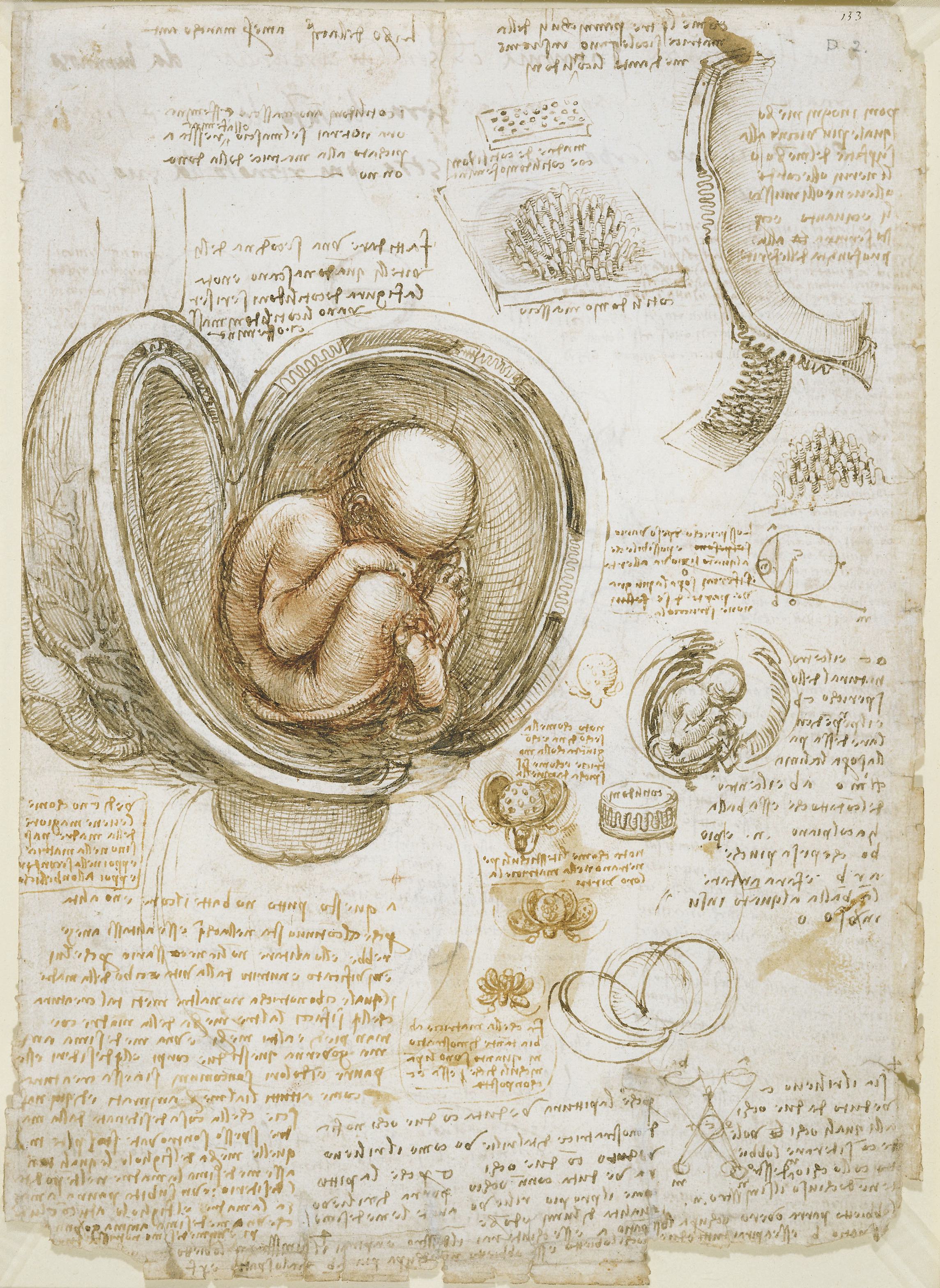
To find another investigator who combined Cajal’s technical ingenuity, deftness with a pen, acuteness of perception, and perseverance, you’d have to go back to Leonardo da Vinci, the first person to systematically document human anatomy. Therein lies the burden of Cajal’s legacy.
The seeming precision of Cajal’s drawings gives them immense narrative power. They were the key weapons he used to overthrow the old reticular theory, which depicted the brain as a single massive network, in favor of his neuron doctrine. This success brought him the 1906 Nobel Prize in physiology or medicine. (Ironically, Cajal shared the prize with the Italian pathologist Camillo Golgi, who had invented the staining methods Cajal used to isolate neurons but who remained a lifelong proponent of the reticular theory.)